Application of design controls to design process. Design review should... | Download Scientific Diagram

9 Tips for Addressing the Documentation Burden of the FDA's Design Control Regulation - Medical Design Briefs

US FDA 21 CFR 820.30 (Documentation and Process for Design Controls For Medical Devices) | Operon Strategist | Isometric, Medical, Online registration

Design Controls: Building Objective Evidence and Process Architecture to Meet FDA and ISO Compliance - OMTEC 2018 | PPT
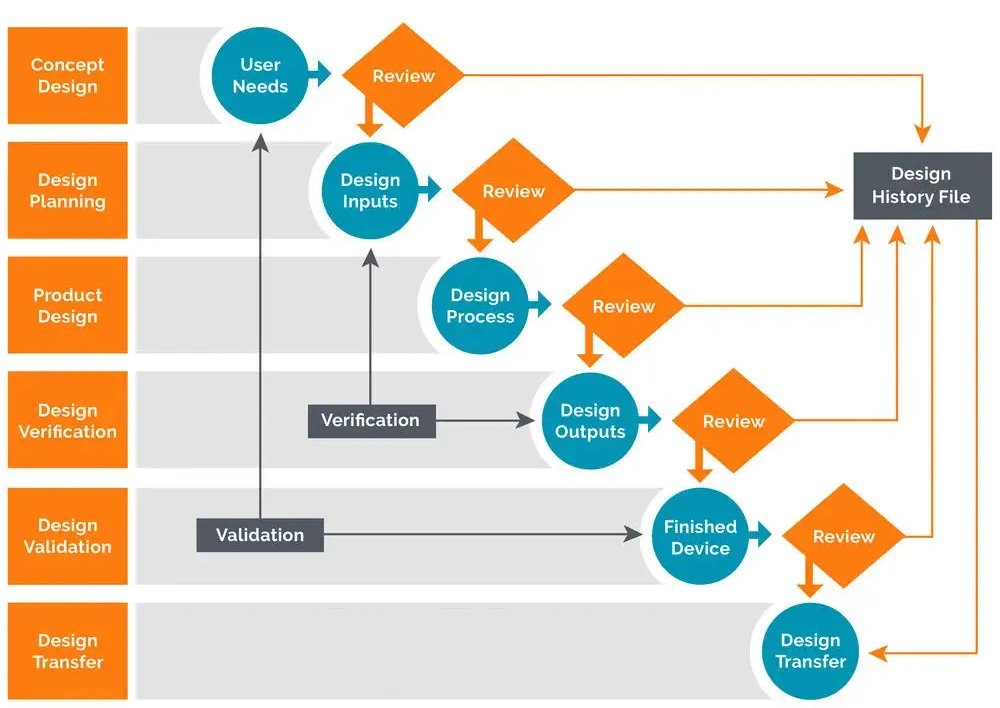
What is Design History File? Why it is Important for Medical Device Development | Medical Device - Johari Digital Healthcare Ltd.

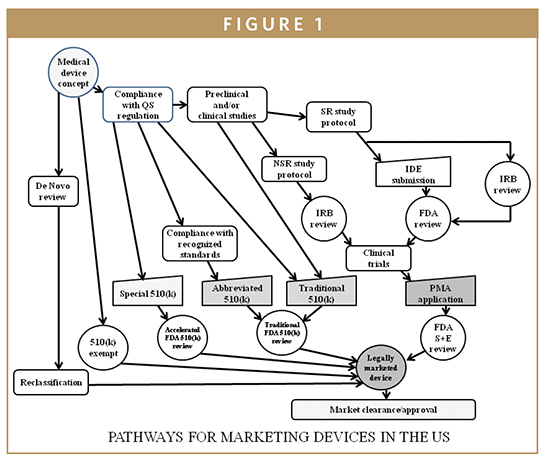
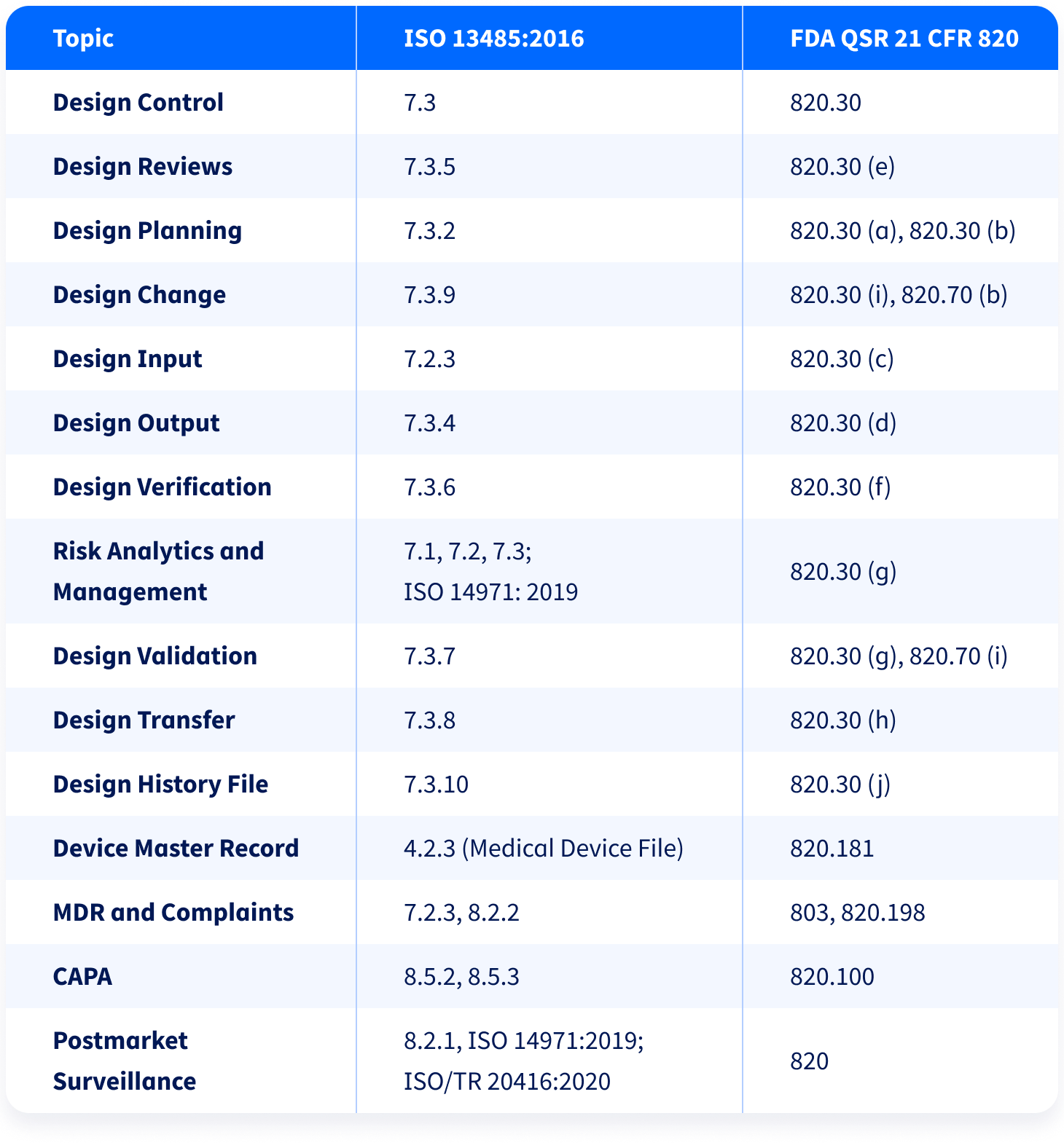
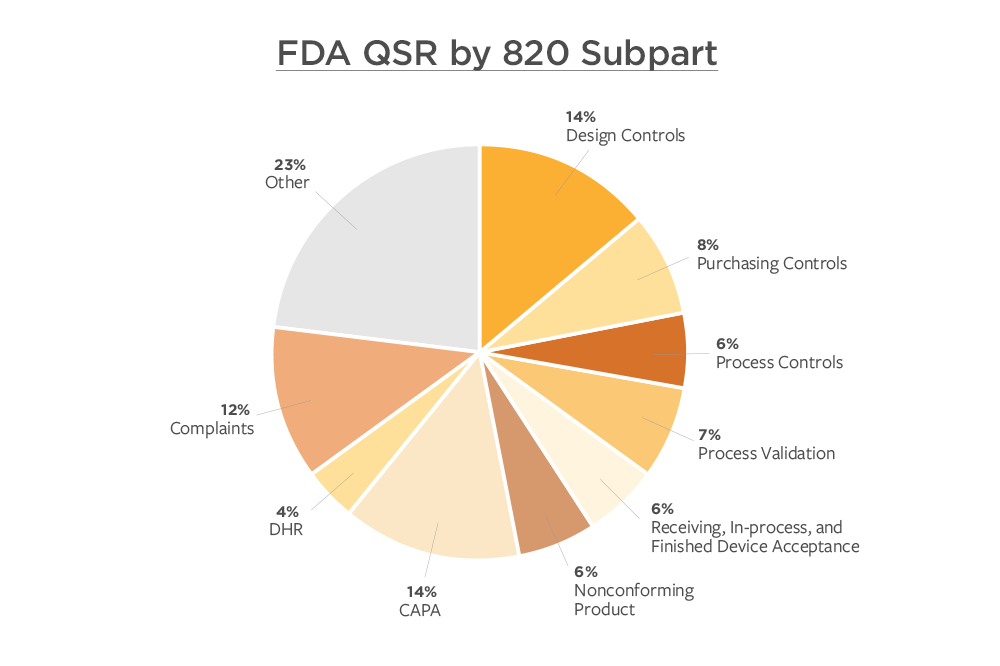


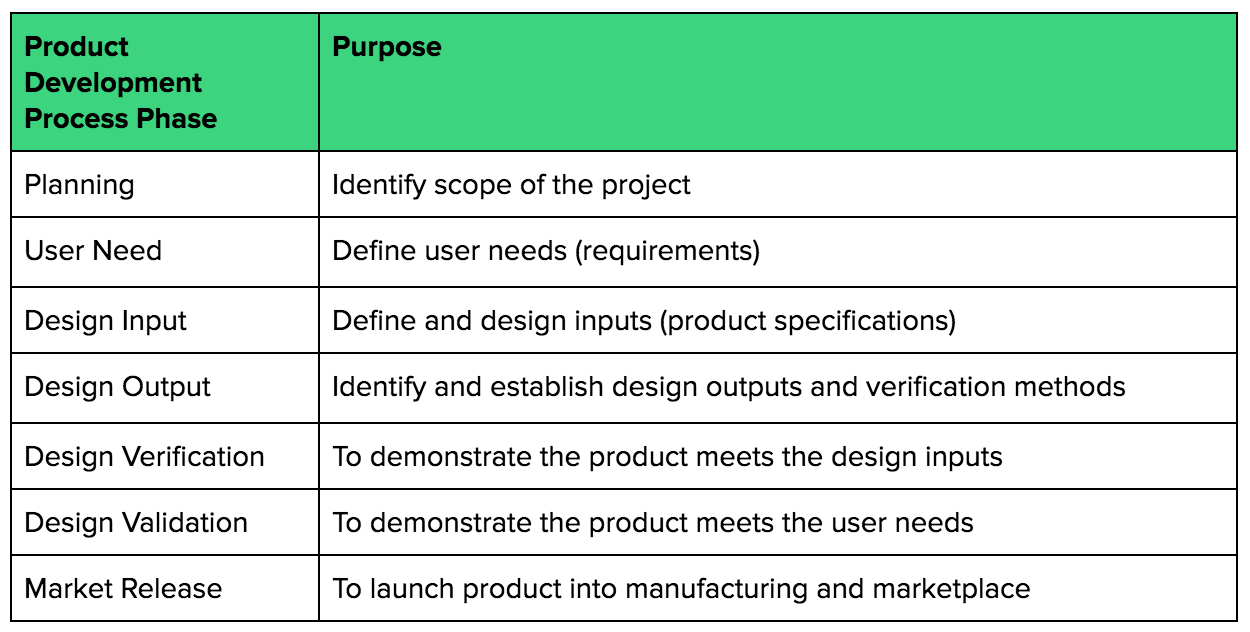



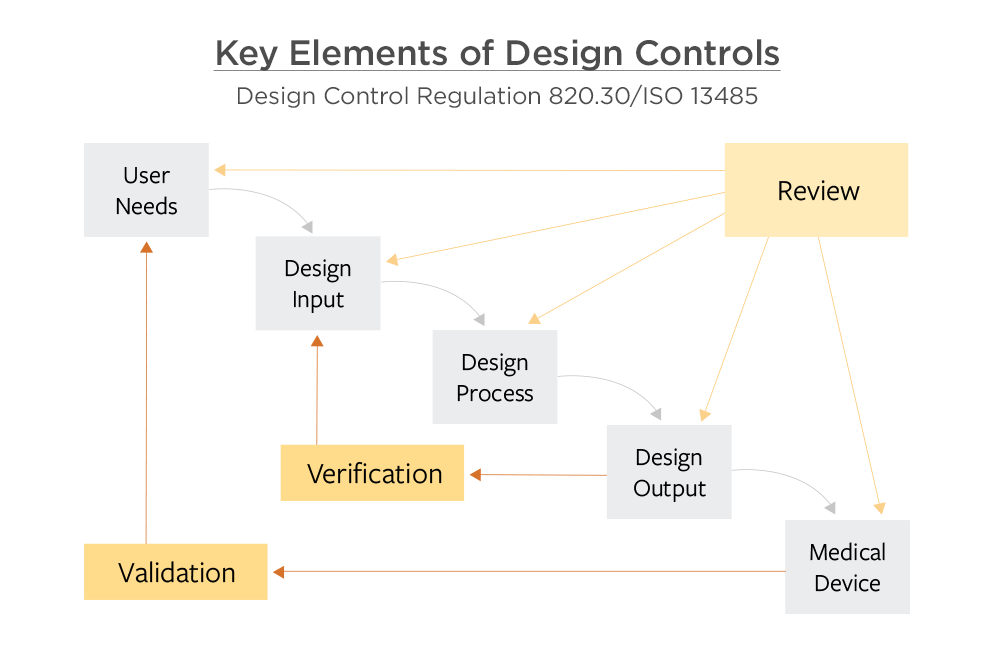


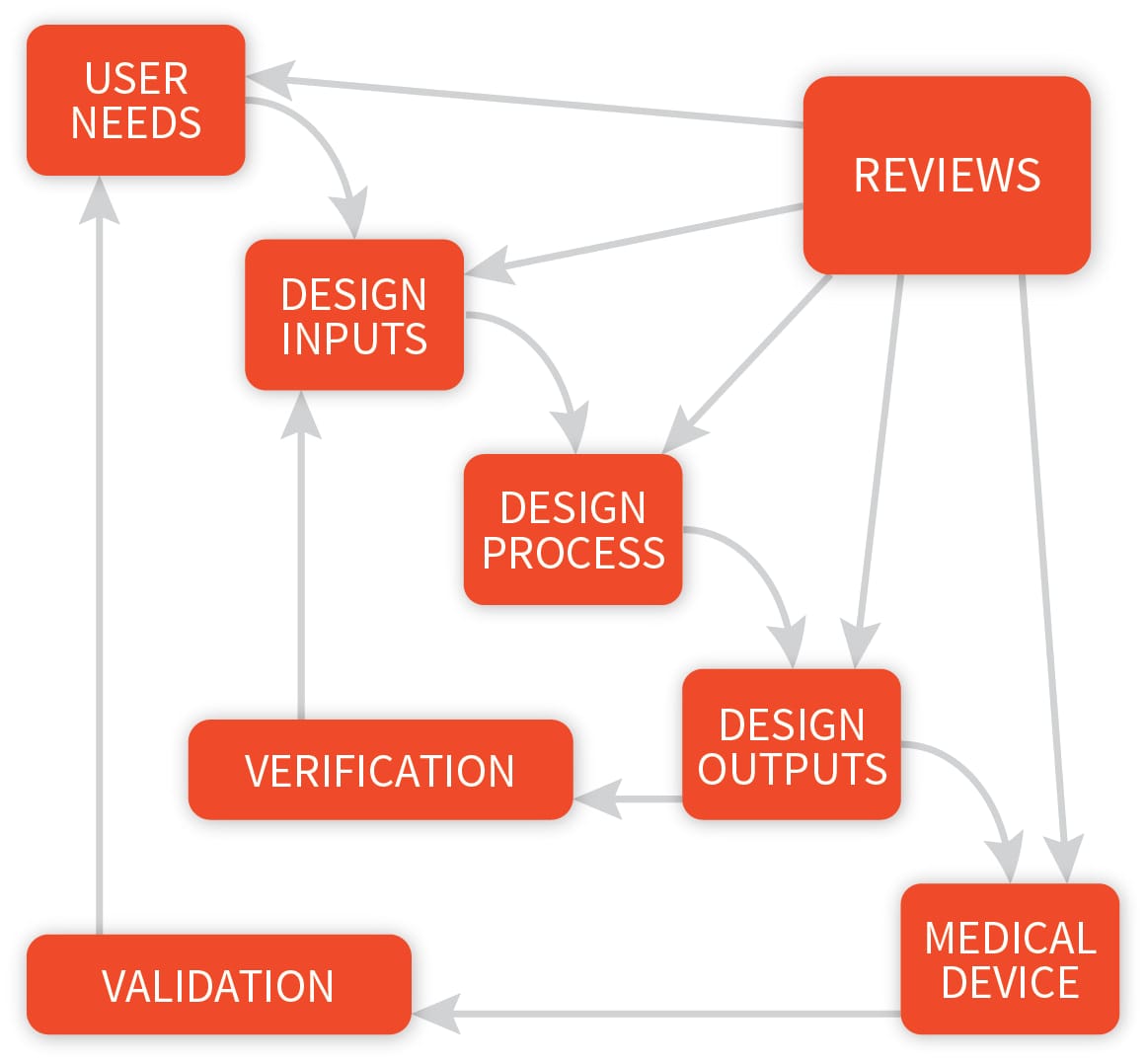

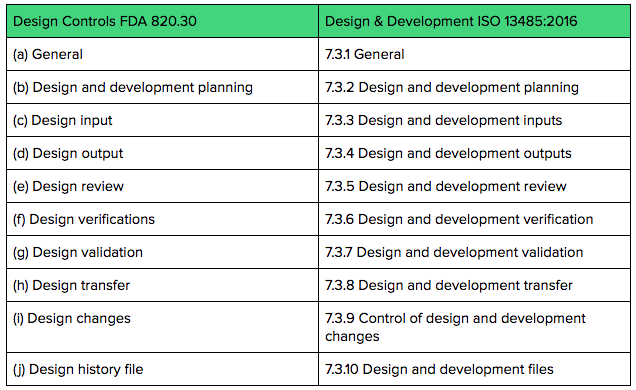
.webp?width=500&height=381&name=Quality%20management%20system%20product%20development%20(1).webp)